Mayoly Spindler announces that it has entered exclusive negotiations with IPSEN (IPN) to acquire its Consumer Healthcare branch, which includes established brands such as Smecta®, Forlax® and Tanakan®, recognized by practitioners and patients worldwide, and distributed in more than 80 countries.
This acquisition would be in line with Mayoly Spindler’s development strategy. A French, family-owned and independent pharmaceutical group, Mayoly Spindler seeks to become one of the world’s leading gastroenterology and dermocosmetics companies through this acquisition. The company has built its development around specialties that are also recognized worldwide, such as Meteospasmyl®, Probiolog® or Topicrem®, many of which are complementary to Ipsen CHC’s therapeutic solutions.
This transaction would accelerate Mayoly Spindler’s development and strengthen its long-term ambition of being a top ten European OTC drugs players as well as becoming part of the top 5 European OTC gastroenterology drugs provider over the next 10 years. With 2,200 employees across all continents, the new entity would benefit from a strong industrial footprint in France, portfolio complementarities in its therapeutic areas, unique commercial synergies and pooled investments in research and development.
“We are proud to be retained by Ipsen as the acquiror of their CHC business, and we look forward to welcoming their employees in order to build together a leading player in consumer healthcare, relying on world-renowned pharmaceutical specialties, a strong industrial footprint in France and a global presence,” said Jean-Nicolas Vernin, Chairman, and Nicolas Giraud, CEO of Mayoly Spindler.
In addition to the support of the historical banking partners, Capza and Caravelle, two French and European financial partners specialized in operations of this scale, will supply part of the funds required to complete this transaction, by acquiring a minority stake in the combined entity.
“We are particularly pleased to support this ambitious growth project in an industry we know well, and to contribute to the creation of a French champion in consumer healthcare”, commented Lorène Martel, President of Caravelle, Guillaume Basquin and Frédéric Chiche, Partners and Co-Heads of Capza Expansion.
The proposed acquisition will be subject to the consultation of employee representative bodies, as well as the finalization of the legal documentation and the customary authorizations, in accordance with the regulations in force. Mayoly Spindler expects to complete the acquisition in the third quarter of 2022.
***
Media Contacts
Florian Ridard / %66%6C%6F%72%69%61%6E%2E%72%69%64%61%72%64%40%76%61%65%2D%73%6F%6C%69%73%2E%63%6F%6D / +33 7 69 59 14 95
Alexis de Maigret / %61%6C%65%78%69%73%2E%64%65%6D%61%69%67%72%65%74%40%76%61%65%2D%73%6F%6C%69%73%2E%63%6F%6D / + 33 6 13 62 38 23
***
About Mayoly Spindler
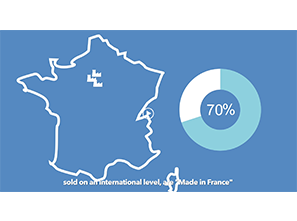
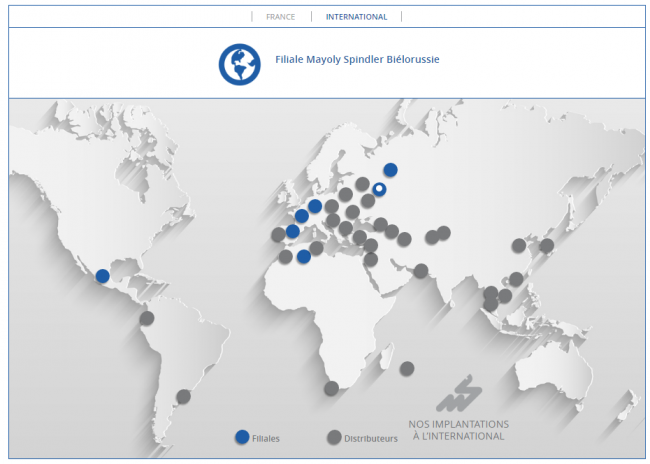
Founded in 1909, Mayoly Spindler is a French, international and independent pharmaceutical company, specialising in Gastroenterology and Dermo-cosmetics. With over 50% of its turnover generated internationally, Mayoly Spindler is continuing its development which started over 110 years ago. With their own production facilities, Mayoly Spindler is able to manage its own industrial developments. Therefore, 62% of the products it sells are made in France. Mayoly Spindler values entrepreneurship, pragmatism and accountability within a caring organisation. All Mayoly Spindler teams work together to build the future of the company and contribute to its global ambitions. Mayoly Spindler is a health entrepreneur committed to supporting patients every day in their well-being and health. To learn more, please visit: https://mayoly.com/en/ or follow us on Twitter and LinkedIn.
About Ipsen CHC
Ipsen’s purpose in consumer healthcare is to bring people across the world care and comfort in their daily lives with healthcare solutions they can trust. Ipsen Consumer HealthCare develops and markets a wide range of innovative solutions – drugs, medical devices and probiotics – in four therapeutic areas: gastroenterology, cognitive disorders, pain, cough and cold. Ipsen Consumer HealthCare is present in 80 countries and employs 1,200 people.
Arab Health 2018
During 4 days, International & Business Team managed our booth at Arab Health, the largest gathering of healthcare/Pharma in the MEA region. The 2018 edition included more than 4,200 exhibiting companies and more than 10,000 attendees from 150+ countries (mainly from MEA region). Our partner in Emirates & Saudi Arabia, Gulf Drug, was gold sponsor of the event, and we received very good feedback of their expertise in the Region.
Mayoly booth was based on French Pavillon even if our neighbour were from Belgium ! Was highlighted the Topicrem products, the HP Franchise with 1 KIBION DYNAMIC & 1 HELIPROBE, the IBS family and the Probiotics franchise, in addition to all others products of the Mayoly.
Supporting for a part the Contract Manufacturing activities (and Calcidose received a very good feedback !), the main objective was to (1) to find new products, and (2) to find new partners for new countries.
After around 200 business cards exchanges, and 4 exhausting days, the conclusion is very positive. We met a lot of Pharmaceutical companies & distributors from Emirates, Saudi Arabia, Iran, Irak, Syria, Jordan, Kuwait, Oman, Bahrain, Yemen, for Middle East, or Kenya and Sudan for Africa for example.
Products presentation, company introduction have been done already, and the follow-up of this event is now crucial, to be able to valuate these 4 days, by new business openings. Our key products have been plebiscited, but some others products, not targeted as priority, may have some potential based on the number of interests received (Colchicine, Calcidose, Cholurso, and the suppository range).
We hope now that business cases arriving in the coming months will be approved to increase our business level in this part of the world, where market growth is very interesting.
We already took an appointment for next year!
 |
 |
Discover our company in 2min30
Discover the first institutional Motion Design of our company !
In 2min30, this graphic animation presents in a synthetic way all the activities of Mayoly Spindler.
New website design for our production unit !
Our Laboratoires Galéniques Vernin (LGV) website has been changed : www.lgvfrance.com !
As a softgel capsules expert since the ealy 1990s, LGV is the production unit of Mayoly Spindler.
Kibion wins the Swecare Export Award 2017
We are pleased to inform you that Kibion, entity of Laboratories Mayoly Spindler, received the SWECARE Award on 26 April 2017 for its outstanding export performance.
The jury that awarded the prize, said :
“By adopting a clear business strategy, Kibion has increased its sales in 2016 by 39% compared to 2015”.
The purpose of the award is to encourage and reward Swedish companies which have an innovative and creative international policy in the field of health.
On 26 April 2017 in Stockholm at the annual Swecare congress, Petter Bäckgren received the prize from Agneta Karlsson, State Secretary for Public Health and Gabriel Wikström Sport Minister.
Swecare is an organization where the academic community and the public and private sectors work together to promote internationally the Swedish healthcare system and the companies involved.

Exhibition EuroPLX
As every year, our laboratory visited EuroPlx, a business development and licensing Exhibition held in Cascais, Portugal, welcoming about 244 companies represented in 44 countries around the world.
https://www.europlx.com/euroPLX-66
Appointments at Mayoly Spindler
Mayoly Spindler expands its management team to support the implementation of an ambitious development cycle
As the French and international world of healthcare faces major changes, Mayoly Spindler, committed to a trajectory of growth, is expanding its management team and consolidating its organization to ensure the soundness and vitality of the components of its success.
“In the five years to come, more than half of our growth will come from new products or new countries and our ability to prepare and carry out these launches is crucial. For our pharmaceutical business, France represents—and will continue to represent—around half of our sales revenue. Under increasing strains and facing ongoing redefinition, it is essential that we have a strong framework in France, capable of sustaining its commercial competitiveness, building the future by fostering the development of new professions and know-how, and contributing to Mayoly Spindler’s strategic thinking”, reports Stéphane THIROLOIX, CEO of MAYOLY SPINDLER.
Stéphane Thiroloix integrates Leem’s board of directors
During their meeting on 6 December 2016, the Leem Board of Directors (Les Entreprises du Médicament) [The pharmaceutical Industry] appointed a new director, Stéphane Thiroloix, General Manager of the Laboratoires Mayoly Spindler, a “Medium-sized French Laboratory”.
Stéphane Thiroloix is a graduate of the HEC (École des Hautes Études Commerciales [Paris Business School]).
He began his career in 1987 at Roussel Uclaf (now Sanofi), where, for eleven years, he held roles in Marketing/Sales and General Management in South Africa, Mexico, Australia and France.
In 1998, he joined SmithKline Beecham (now GlaxoSmithKline), where he was appointed Vice-President and Director of French Operations, and then Vice-President for Europe in Business Development and Marketing Alliances.
From 2002 to 2007, Stéphane Thiroloix was Vice-President of French Operations and then became Vice-President for Europe and General Manager for France at Bristol-Myers Squibb.
In 2007, he joined the Ispen company’s executive committee as Executive Vice-President of Corporate Development and held this position until 2011, the year he became President of Smith & Nephew’s Advanced Surgical Devices (ASD) Division for Europe, Canada, Japan and Australia.
In 2014, Stéphane Thiroloix became General Manager of Laboratoires Mayoly Spindler.
A new subsidiary in Belarus
News BabySpasmyl®
The BabySpasmyl® dosage:
Infants: 20 drops (around 1 ml) 1-2 times a day
Children: (20 drops, 1-3 times a day)
Adultes : 20-40 gouttes 2-4 fois par jour
BabySpasmyl® is a simethicone-based medical device with physical and mechanical actions, which helps in relieving infant colic, aerophagia, bloating and gastrointestinal disorders in adults, children and infants. It is manufactured by NTC. Read the package leaflet carefully before use. In case of doubt, ask your doctor or pharmacist. This medical device is a regulated healthcare device that, in accordance with this regulation, has the (CE 0426) CE marking, issued by ITALCERT. November 2016.